Discusses the development of aluminum air fuel cells at home and abroad in recent decades. The characteristics of the aluminum air fuel cell, its working principle and the research progress of the entire battery system are mainly analyzed. The market application and development prospects of aluminum air fuel cells are discussed.
Aluminum air fuel cell is a new high-energy chemical power source [1]. The battery has the advantages of large energy density, light weight, rich source of materials, no pollution, high reliability, long life, and safe use [2-4]. Therefore, it stands out among many batteries and is generally favored by all countries in the world. The United States, Canada, the former Yugoslavia, India, Norway, the United Kingdom, and Japan are all actively conducting research. Due to the successful development of good performance air electrodes, great progress has been made in the research of aluminum air fuel cells. Foreign countries have made great progress in terms of medium, high power, or low-power aluminum air fuel cells [5]. Relatively speaking, China started relatively late and there are not many research institutions. Among them, Harbin Institute of Technology began research on aluminum air fuel cells in the 1980s, completed research on anode quaternary aluminum alloys in 1983, and completed sample development of 3W neutral aluminum air fuel cells in the 1990s[9]. A 1kW alkaline aluminum air fuel cell stack was developed [7]. In the early 1990s, Tianjin University successfully developed a marine high-power neutral electrolyte aluminum air fuel cell stack, and has been engaged in the study of low-power neutral electrolyte aluminum air fuel cells for electric vehicles [8-10]. The battery is entering the commercial application stage. In the 1990s, Wuhan University also made a preliminary study on seawater aluminum air fuel cells [11].
However, there have been fewer and fewer research institutions in recent years and there have been very few related reports. Therefore, this paper analyzes and reports on the current situation of foreign aluminum air fuel cells.
1 The working principle of aluminum air fuel cell
Aluminum air fuel cells use high-purity aluminum (aluminum content of 99.999%) or aluminum alloy as anode, oxygen (air) electrode as cathode, and alkali or salt as electrolyte. During the discharge, the anode dissolves and oxygen in the air is reduced to release electrical energy. According to the electrolyte, it can be divided into basic (electrolyte is alkali) and salt (electrolyte is salt, usually salt water) aluminum air fuel cell. Aluminum is the most abundant metal element on the earth, accounting for the third element in the distribution of elements, and the global industrial reserves of aluminum has exceeded 2.5×1010t. For a century, aluminum has been the largest and most widely used non-ferrous metal in the world. In 1996, the total global output reached 1.7×107 tons [12]. Therefore, aluminum is rich in anode materials. Aluminum is a kind of active metal, it is more attractive than metal zinc, magnesium and the like. Because the electrochemical equivalent of aluminum is very high, it is 2980Ah/kg, the electrode potential is negative, and for the metal with the highest specific energy except lithium, the mass specific energy of aluminum air fuel cell can reach 450Wh/kg, and the volumetric energy is less than lead acid. The battery has a specific power of 50-200 W/kg [13]. Aluminum emits 3 electrons per atom, while zinc and magnesium release only 2 and lithium releases 1 atom.
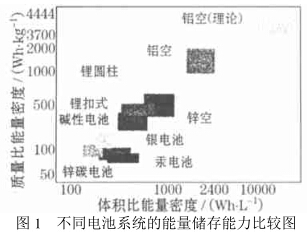
That is to say, the amount of raw material needed to produce the same amount of energy, aluminum is the least. Therefore, many factors combine to make aluminum the best choice for anode materials for metal air fuel cells. Figure 1 shows the comparison of aluminum air fuel cells with other primary battery systems: zinc air, lithium cylinder, lithium ion, alkaline, silver, mercury, and zinc-carbon batteries. The figure shows that aluminum air fuel cells have the highest mass ratio. The energy density and volumetric energy density are consistent with the extremely high specific power density of aluminum air fuel cells.
Different electrolytes, aluminum air fuel cell reaction mechanism is also different.

Due to the corrosion reaction of aluminum metal, hydrogen gas is generated. Therefore, the battery system must be handled safely, and vent holes must be reserved.
The difference between aluminum air fuel cells and alkaline aluminum air fuel cells under salt conditions is mainly reflected in the reaction products, voltage and power. Under salt conditions, the low voltage is suitable for low and medium power applications, while under alkaline conditions, high voltage, can be applied to low power, can also be applied to medium and high power applications such as electric car power. Under salinity conditions, the reaction product is an insoluble gibbsite gel, and by adding a special inhibitor to the electrolyte, the colloid is peeled off from the anode in the form of crystallized powder, thereby not affecting the battery reaction. Under alkaline conditions, the reaction product is soluble Al(OH)-4 has no precipitation problem, so it is much simpler in design than the salt aluminum air fuel cell, and the auxiliary facilities are much less.
2 Research Progress of Aluminum Air Fuel Cell
2.1 Research progress of aluminum anode
Aluminum air fuel cells use high-purity aluminum or aluminum alloys as anode materials for batteries. In essence, aluminum is resistant to corrosion, and an Al2O3 protective film is naturally formed on the surface thereof, which suppresses the oxidation and electron-loss reaction of aluminum. Due to the presence of surface oxide films, most of the aluminum potential is lost due to anodic polarization even under open circuit conditions [14].
Under alkaline conditions, the aluminum anode is easily corroded. Under open circuit conditions, the corrosion rate will gradually increase as the anode dissolves. At the same time, the presence of electrochemical local cells in the aluminum impurities, so that the corrosion reaction of aluminum in aqueous solution will be accompanied by the generation of hydrogen, proposed a safe handling problem [15]. Therefore, for applications in fuel cells, aluminum must exhibit very good electrochemical activity in battery reactions, ie low corrosion rate, low anode overvoltage and low anode corrosion potential [16]. The above problems can generally be solved by alloying high-purity aluminum (99.999%) with other superior metal elements [17].
Now it has been developed from the binary alloy to the addition of seven yuan or even more, so that the aluminum anode performance greatly improved. The purpose of adding these elements is to reduce the autolysis rate of the anode, improve the polarization properties of the aluminum, and improve the electrochemical performance of the anode. China Harbin Institute of Technology completed research work on quaternary aluminum alloys as early as 83 years ago and successfully applied to the research of 3W aluminum air fuel cells.
In the 1990s, a new type of five-membered aluminum alloy was successfully developed. The alloy has excellent properties, and the aluminum electrode has a relatively negative potential in a wide current density range. In foreign countries such as India, the research conducted by American Electric Technology Research Corporation and Canadian Aluminum Corporation (established in 1998) has made great progress in this area. The quaternary alloys developed in India in the past 87 years are very good. The quaternary alloy composition is Al-4%Zn-0.025%In-0.1%Bi, the corrosion rate is 0.498mgcm-2min-1, and the open circuit voltage is -1.450V. The open circuit voltage was -1.157V at 150mAcm-2, and the anode efficiency was 97% at 150mAcm-2. And its alloy is only based on commercial aluminum with a purity of 97%. If it is a high-purity aluminum alloy, its performance will be superior [18].
By adding a small amount of alloy formed of aluminum, such as indium, manganese, magnesium, silver, niobium, tantalum or niobium, the aluminum anode can have high performance. At present, 99.999% of high-purity aluminum is generally used. The impurities in the alloy are rarely in the range of 10-5, and the effect is negligible. For alkaline aluminum air fuel cells, corrosion inhibitors have been added to the electrolyte. Currently, a single corrosion inhibitor such as citrate, stannate, In(OH)3, K2MnO4, CaO, BiO-3, Ga(OH)-4, etc. has been developed into a composite corrosion inhibitor such as Na2SnO3+In(OH). 3, citrate CaO, etc., very good to reduce the anode's self-corrosion. India has more research on corrosion inhibitors and more mature technologies. In the 1980s and 1990s, there were more reports [19–22]. The effects and mechanisms of different corrosion inhibitor systems on the behavior and corrosion rate of different grades of aluminum anodes and their alloy anodes were systematically studied. For neutral aluminum air fuel cells, the gelatinous substance gibbsite produced by elimination of the reaction by adding a special inhibitor to the electrolyte has been developed. Due to the formation of a gel-like substance, it adheres to the surface of the anode, preventing the electrode reaction, thereby reducing the reaction rate and anode efficiency.
By adding a special inhibitor such as SnO-23 [19] in the electrolyte and using the inhibitor as the nucleus, the gibbsite is present as a crystalline powder, which naturally precipitates on the bottom layer of the electrolyte, thus eliminating the coagulation The bad effects of the glue material increase the reaction rate and make the aluminum anode perform well. FIG. 2 is a practical application of an aluminum air fuel cell and its aluminum anode plate. The shape of the anode plate is approximately wedge-shaped, which is consistent with the mechanism of the consumable wedge-shaped anode [23] studied by Professor ARDESPIC in the former Yugoslavia, which improves the utilization of the aluminum anode. In order to optimize the performance of aluminum anodes, dome-type aluminum anode plates have been developed [24]. Seawater aluminum air fuel cells using this shape of aluminum anode plates have the following properties: a constant anode surface area during discharge; always The constant anode-cathode distance; the smallest anode surface area to volume ratio minimizes self-discharge; while the reacted gel product can be washed away by seawater at any time without sticking to the cell surface. The successful development of this kind of electrode makes the practical application of aluminum air fuel cell under the sea become a reality.
In summary, the current research techniques of aluminum anodes in various countries are relatively mature, and there are also many studies on the rational ratio and mechanism of high-purity aluminum and its alloys. For example, the report on the progress of aluminum alloy anode activation mechanism by Tianjin University [13] has practical significance. These will be very beneficial to the development of aluminum air fuel cells.
2.2 Cathode Research Progress
The high development of aluminum air fuel cells benefits from the research of high-performance oxygen (air) electrodes. The role of the oxygen electrode is to reduce oxygen. The characteristic is that there must be catalyst when reducing oxygen. The principle of electrochemistry is as follows:

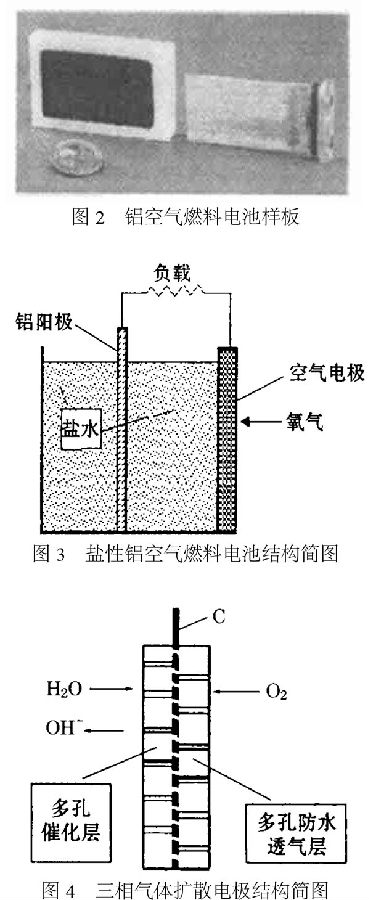
Theoretically, some transition elements can be used as good catalysts. [25] In practice, silver, platinum and manganese are proved to be effective catalysts for the reaction. Since the solubility and the diffusion rate of oxygen in the aqueous solution are small, the current density of the two-phase electrode is small. With the development of organic bonding materials such as Teflon and advanced film-making techniques, three-phase gas diffusion electrodes have been used [26]. FIG. 3 is a schematic view of a salt aluminum air fuel cell.
A schematic diagram of the structure of this three-phase gas diffusion oxygen electrode is shown in FIG. 4 .
Mainly consists of waterproof breathable layer, catalytic layer and conductive layer.
The waterproof breathable layer is mainly composed of hydrophobic material PTFE, and a pore forming agent such as Na2SO4 is added to form a large number of capillary pores therein, thereby preventing electrolyte leakage and allowing air to enter the electrolyte through the pores.
The catalytic layer is composed of a catalyst and its carrier, polytetrafluoroethylene, and is the site of electrode reactions. Oxygen is reduced here.
The conductive layer is a cathode electron current collector and can also increase the mechanical strength of the cathode, typically a nickel mesh or a nickel-plated copper mesh.
U.S. Electric Technology Research Corporation and Alcan Canada have focused their research on the catalysts and electrode structures of oxygen (air) electrodes, and have made new advances. High-power automotive power batteries have been put to practical use. According to reports, Alcan’s aluminum air fuel cells can be used to increase the operating distance of automobiles from 75km to 300km compared to lead-acid batteries. The energy density is more than 7 times that of lead-acid batteries, and the space occupied is only lead-acid batteries. /7.
As the oxygen (air) electrode research has always been a bottleneck restricting the development of aluminum air fuel cells. Therefore, the research of high-performance oxygen air electrode plays an important role in the deep development of aluminum air fuel cells.
3 Aluminum Air Fuel Cell Application Research Progress
Although aluminum air fuel cells have achieved great development, they have not yet been commercialized. The main reason is that some related technologies are still not mature enough and there are still some problems that have not been solved. General medium and high power large aluminum air fuel cell stacks or battery stacks require an air circulation system and an electrolyte circulation system. For the air circulation system is mainly how to reduce the CO2 in the air to eliminate the generation of carbonate on the air electrode and improve the electrode performance. Although silver and platinum have a good catalytic effect, there are problems with catalyst poisoning and failure, and the price is also relatively expensive, which is also a major obstacle to commercial application. At the same time, there is a problem of cobalt loss in the cobalt tetramethoxy porphyrin complex and the design problem of the air circulation system. Therefore, it is necessary to develop new catalysts with good quality and low cost, such as MnO2, and the use of nanotechnology is also an important research topic. Although a special inhibitor can be added to the electrolyte circulation system to precipitate gibbsite crystals so that the electrolyte can be used after separation, the separation device and its process are still unknown, and there are no systematic studies. What's more, domestic research on aluminum air fuel cells is rarely reported, so it is far behind foreign research on aluminum air fuel cells.
At present, foreign countries have made remarkable achievements in how to solve the above problems. This makes the application of aluminum air fuel cells more and more extensive. As early as in the 1980s, electric vehicles powered by aluminum air fuel cells made in California, the United States, can travel 1,600 km with one additional fuel. An Al-air fuel cell vehicle developed by Professor Ardespic of the Belgrade University of the former Yugoslavia and supplemented with an aluminum electrode can also travel 1600 km [27].
Being a power source for automobiles is a major application of aluminum air fuel cells. At present, Canadian Aluminum has made great progress in this regard. The company's automotive power supply has the same performance as mentioned above.
As an offshore and military application, aluminum air fuel cells also demonstrate its superior performance advantages. The sea aluminum air fuel cell developed in the United Kingdom in 1994 was made of PTFE Co3O4/C oxygen reduction cathode. The battery was operated in the subsea environment for more than one year. The energy density was 1008Wh/kg[28], which was much higher than other primary batteries. system. Therefore, the application prospects in this field are extensive.
The other major application of aluminum air fuel cells is also an application widely used by news and media as a portable power source for mobile phones and laptops. Also known as 'personal power'. It is reported that the energy density of the aluminum air fuel cell is more than 75 times that of a lithium ion battery. Alcan Canada has achieved great research results in this area. At present, the company has announced a new product, which will allow the mobile phone to have a talk time of 8 hours and a standby time of nearly 6 days [29]. The company is committed to optimizing its products for 25 hours of talk time and more than 10 days of standby time. At present, Canadian TrimolGroup Company stated that the company will launch the company's new generation of aluminum air fuel cells in the spring of 2002, enabling mobile phone talk time of 24 hours or backup time to reach one month. The cell phone battery power will be 13 times that of the same size lithium-ion battery. Moreover, the company's aluminum air fuel cells for laptop computers are expected to be used for 12h to 24h, while lithium-ion batteries are generally used for 30min to several hours. Figure 5 is based on Nokia 6000 series used in cell phone battery performance comparison chart, the figure shows that the aluminum air fuel cell has the best capacity performance 12000mA · h, while the zinc air battery and lithium ion battery capacity is 3500mA · h, 900mA · h .
Table 1 shows the portable aluminum air fuel cell design of laptop computer designed by Trimol Group of Canada. From the table, it can be seen that whether it is from the battery capacity, or from the actual size and working environment conditions, the battery is The performance is very superior. Therefore, the application prospects of aluminum air fuel cells in 'personal power supply' will be unprecedented, and future development is inevitable.

Other potential applications are areas where there is no electricity, insufficient power, or high electricity prices. Aluminum air fuel cells can meet the requirements. It can also be used as an alternative to an internal combustion engine generator to eliminate air pollution. Voltek, a foreign company, mainly develops portable power supplies. The company's products, called 'FuelPak', are designed and manufactured for emergency power supplies, submarine operations, and power supplies such as forklifts.
For the market outlook of aluminum air fuel cells, Trimol expects the following. By 2006, there will be 1.4 to 1.8 billion mobile phone users in the world, which will consume 1.8 to 2.16 billion batteries. The sales of mobile phone batteries will reach 1080 billion U.S. dollars, and metal air fuel cells will account for 6% of the market; In 2015, there will be 151 million portable camcorders worldwide, 2.58 billion US dollars in the market, and metal air fuel cells will account for 12% of the market. At the same time, laptop computers provide another more attractive market for the development of aluminum air fuel cells. By 2006, there will be 240 million laptops worldwide, and metal air fuel cells are expected to occupy 4% of the market. Therefore, the market prospects for metal air fuel cells are broad, and will make even greater breakthroughs in the next few years.
The development of aluminum air fuel cells in China is of great significance and the market is extensive. In the area of ​​electric vehicles, there are numerous automobiles in major cities in China, and there is a serious air pollution. This raises the necessary issues for the development of aluminum air fuel cells, and we should vigorously develop ecological vehicles. On the other hand, the rapid development of China's mobile communications industry, the mobile phone battery market, and the rapid increase in the number of personal computers, it is imperative to develop efficient batteries. Comprehensive development of all countries in the world, combined with China's actual national conditions, it is necessary to vigorously develop aluminum air fuel cells in China.
references:
[1]LiJinghong.Metal/airfuelcell[R].21stCenturyFuelCellTechnologyInternationalForum, 2001.190-191.
[2] Select Quasi-batteries to Avoid Detours [J]. Guangdong Agricultural Machinery, 1998, 3:7-9.
[3]Gregory DP. Metal-air batteries (top) [J]. Batteries, 1985, 50(1): 20-32.
[4]Gregory DP. Metal-air batteries (below) [J]. Batteries, 1985, 51(2): 20-29.
[5] Status of thealuminum/airbatterytechnology [J]. Elec-trochemSoc, 1992, 11:584-598.
[6] Shi Pengfei et al. Research on three-watt aluminum-air battery[J]. Batteries, 1992, 22(4): 152-154.
[7] Shi Pengfei et al. Study on 1-kilowatt aluminum air battery [J]. Power Technology, 1993,1:11-17.
[8] Jiang Taixiang, et al. Process Study on Oxygen Electrolyte Catalyst for Al-air Battery [J]. Power Technology, 1994,2:23-27.
[9] Liu Zhihui et al. Design of neutral electrolyte air battery for stationary electrolyte [J]. Power Supply Technology, 1992, 5:6-8.
[10] Liu Zhihui et al. Study on high power static neutral electrolyte aluminum air battery for ship[J]1 Power Technology, 1993, 6:27-32.
[11] Wang Zhendao et al. Aluminum sea air fuel cells [J]. Power Technology, 1997, 21 (3): 106-113.
[12] Zhang Hao et al. Research progress of aluminum chemical power supply [J]. Modern Chemicals, 1998, 10:9-11.
[13] Qin Xue, etc. Aluminum alloy anode activation mechanism research progress [J]. Power Technology, 2000,24 (1) :33-37.
[14]Brown OR, Whitley JS.Electrochemicalbehaviorofa-luminuminaqueouscausticsolutions[J].ElectrochemicaActa,1987,32(4):545-556.
[15] HoriY, TakaoJ, ShomonH1 Aluminumalloysforalu-minumprimarycell[J].ElectrochemicaActa,1985,30(9):1121-1124.
[16] Wilhelmsen W, Arnesen T, Hasvold Q, Stqrksen NJ. The electrochemical catalyst of Al-Inalloysinalkalineelectrolytes [J]. Electrochemica Acta, 1991, 36(1): 79-85.
[17] Shi Pengfei et al. Novel aluminum alloy anode and its electrochemical behavior in alkaline solution [J]. Power Technology, 1992, 6:5-9.
[18] SheikMideenA, GanesanM, AnbuklandainAthanM, etal.Developmentofnewalloyofcommercialaluminumwithzinc,indium,tin,andbismuthasanodesforalka-linebatteries[J]. PowerSources, 1987, 27:233-244.
[19] AlbertHN, AnbukulandainathanM, GanesanM, etal.
Characterisation of differentgrades of commercial sexualpurea-luminumasprospectivegalvanicanodesinsalineandalka-linebatteryelectrolyte[J].AppliedElectrochemistry,1989,19:547-551.
[20]MacdonaldDD, EnglishC.Developmentofanodesfor
Aluminum/airbatteriessolutionphaseinhibitionofcorro-sion[J].AppliedElectrochemistry, 1990, 20:405-417.
[21] Kapali V, Venkatakrishna IyerS, Balaramachandran V, et al.Studieson thebestalkalineelectrolyteforaluminum/airbatteries [J]. PowerSources, 1992, 39:263-269.
[22]GnanaSahayaRosildaL, GanesanM, etal. Influence ofinhibitorsoncorrosionandanodicbehaviorofdifferentofa-luminuminalkalinemedia[J]. PowerSources, 1994, 50:321-329.
[23]DespicAR.Designcharacteristicsofanaluminumairbatterywithconsumablewedgeanodes[J].AppliedElectrochemistry,1985,15:191-200.
[24]ShenPK, TseungACC.Designofadomeshapesalu-minumwaterbattery [J]. Applied Electrochemistry, 1994, 24: 145-148.
[25] Li Chunhong. Application of rare earth elements in fuel cells [J]. Power Supply Technology, 1992, 3: 24-28.
[26]Tang Luencheng et al. Study on the High Efficiency Surface Catalytic Layer of Alkaline Fuel Cell [J]. Power Supply Technology, 1995,19(1):54-57.
[27]DespicAR, Milanovic PD. Aluminum-airbatteryforelectricvehicles[R]. RecTravInstSciencesTechniquesAcademicSerialSciencesArts, 1979, 12(1): 1-18.
[28]ShenPK, TseungACC.Developmentofanalu-minum/seawaterbatteryforsubseaapplications[J]. Pow-erSources, 1994, 47:119-127.
[29] Talk, talk, talk: "greenpower" aluminum/airfuelcellboostsphoneusetimeto8hours[R].FuelCellIndustryReport, 2000.1(1)
[30]Metal/airFCmakesprogress[R]1FuelCellIndustryReport, 2001.2(3)
Filter Disc is processed by puncher with special mould. It's one of the filters; meanwhile it's also one of the most widely used products. The wire mesh disc is mainly based on the processing customized size and shape of the customers. The filter mesh has so many different apertures, that it can meet the needs of different filters. The filter mesh appearance is very beautiful, for a variety of containers is no problem
The shape of Metal Filter Disc include rectangle, square, round, ellipse, ring, rectangle, hat shape, waist shape and abnormity.
Wire mesh disc structure: single, double, multilayer.
Processing technology: double or three layers of spot welding, solder joints are generally 4 - 10 different, also can according to customer requirements to do single-layer and double layer hemming.
Rim material: stainless steel plate, copper plate, galvanized plate, aluminum plate, etc.
Filter diameter: generally 5 mm to 600 mm
Use: Filter wire mesh products (referred to as filter), mainly used in rubber, plastics industry, grain and oil, oil refining, screening, chemical industry, light industry, medicine, metallurgy, machinery, shipbuilding, automobile and tractor industry in distillation, absorption, evaporation, filtration process, eliminate entrained in the steam or the droplets in the gas and liquid foam, and car used as air filter.
Filter Disc
Filter Disc,Stainless Steel Liquid Filter Discs,Stainless Steel Filter Disc,Metal Filter Disc
Anping Xinzheng Metal Wire Mesh Co., Ltd , https://www.sievingmesh.com
