(Source: Qian Cheng, Department of Engineering, Columbia University)
According to foreign media reports, improving battery energy storage capacity, increasing battery life, and ensuring battery safety, it is becoming more and more important to solve the above challenges, because everyone is more and more dependent on mobile devices and electric vehicles that require such energy device. However, on April 22 local time, the engineering team of Columbia University, led by Assistant Professor Yuan Yang of the Department of Materials Science and Engineering, announced that a new method has been developed that can be stabilized by implanting boron nitride (BN) nanocoating The solid electrolyte in a lithium metal battery, thereby safely extending battery life.
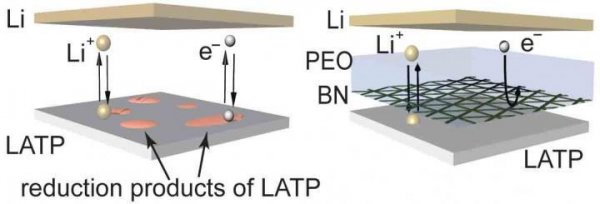
At present, traditional lithium-ion batteries are widely used in daily life. Such batteries have a low energy density, resulting in a short life span, and because the battery contains a highly flammable liquid electrolyte, it may also short-circuit or even catch fire. Replacing the graphite anode in lithium-ion batteries with lithium metal can increase the energy density of the battery; the theoretical charging capacity of lithium metal is nearly 10 times higher than graphite. However, in the process of lithium plating, it is easy to generate dendrites. If the dendrites penetrate the separator in the middle of the battery, it will cause a short circuit, thereby causing battery safety concerns.
Yang said: "We decided to focus on solid, ceramic electrolytes. Compared with flammable electrolytes in traditional lithium-ion batteries, solid ceramic electrolytes show great potential in improving safety and energy density."
Most solid electrolytes are ceramic, so they are not flammable, eliminating potential safety hazards. In addition, the solid ceramic electrolyte has high mechanical strength, which can actually inhibit the growth of lithium dendrites, so that lithium metal can be used as a battery anode coating. However, most solid electrolytes are unstable to lithium ions, easily corroded by lithium metal, and cannot be used in batteries.
To address these challenges, the research team collaborated with Brookhaven National Lab and the City University of New York. The researchers deposited boron nitride (BN) between 5 and 10 nanometers. The nano-membrane is used as a protective layer to isolate the electrical contact between the lithium metal and the ionic conductor (solid electrolyte), and a small amount of polymer or liquid electrolyte is added to penetrate the electrode / electrolyte interface. The researchers chose boron nitride as the protective layer because it is chemically and mechanically stable to lithium and has a high level of electrical insulation. The boron nitride designed by the researchers has holes inside, through which lithium ions can pass, making it an excellent separator. In addition, using chemical vapor deposition to prepare boron nitride, it is easy to form a large-scale (decimeter-level), atomic-like thin-scale (nano-level) continuous thin film.
Researchers are currently extending their methods to various unstable solid electrolytes, and further optimize the interface, hoping to produce high-performance, long-cycle solid-state batteries.
Spider Fitting normally use for glass curtain wall. JIANGYI has specialized in door hinges since 2000. We have many kinds of spider fitting, such as one arm, two arms, three arms, four arms, 180 degree, 90 degree and so on. All of them are in High quality and inexpensive. Furthermore, we can produce it according to your new style.
Specification
1. Material: SUS304, SUS316
2. Finish: Mirror / Matt
3. Suitable for glass thickness: 12-25MM
4. Application: glass curtain wall
5. Must be tempered glass
Spider Fitting
Spider Fitting,Glass Spider Fittings,Spider Glass Fitting,Spider Fittings For Glass
Jiangyi Industrial Co., Ltd , https://www.cnjyhardware.com
