Abstract: The cotton fabric was functionally finished with nano-hydroxyapatite with stable performance. It was used to improve the optical stability of cotton fabric in photocatalysis and the adsorption of nano-titanium dioxide. Methods: The key technology for the preparation of titanium dioxide photocatalytic functional materials based on cotton fabrics was studied. The results show that the nanometer titanium dioxide dispersion system is best prepared by using 1:1 ethanol/water solvent. When the mass fraction of titanium dioxide in the dispersion system is 3%, the decomposition effect of formaldehyde on cotton fabric under ultraviolet irradiation is obtained. optimal.
With the development of photocatalytic materials and the development of application technology, nano TiO2 has a wide range of applications in the textile field, such as the development of anti-UV fiber and antibacterial fiber, but the use of nano-photocatalytic materials for the functional finishing of fabrics. Quite a number of theoretical and application issues remain to be studied. It is mainly reflected in the loading technology of nanomaterials on textiles, the influence of photocatalytic materials on textile properties and the catalytic oxidation performance of functional textiles. Cotton fiber is an important textile material. The application of nano-photocatalysis technology to the functional finishing of cotton fabrics and the development of cotton decorative products with air purification function can not only improve the value of cotton fabrics, expand the application range of cotton fabrics, but also explore nanometers. The application of photocatalytic materials in the field of organic polymer materials has guiding significance.
Starting from the molecular structure of cotton fiber, this paper cites an inorganic finishing agent which can be hydrogen bonded with cotton fiber, has stable performance and has no side effects on human body. Nano-hydroxyapatite is used as a protective agent for cotton fiber, which effectively improves. Optical stability of cotton fibers and load capacity of photocatalytic materials on cotton fibers. In order to develop cotton decorative products with air purification function (using photocatalytic decomposition of formaldehyde, odor and other substances), this paper further based on the formaldehyde decomposition effect, through the single factor test and multi-factor orthogonal test analysis, the protective agent treatment Cotton fabrics were studied on the surface TiO photocatalyst deposition process in order to find the best cotton fabric treatment process.
1 test part
1 . 1 test material
Pure white cotton knit fabric; self-made nano hydroxyapatite (HAP), chemical formula Ca 10 (PO4) 6 (OH) 2 , particle size of about 30nm; titanium dioxide (TiO2), P 25 nanometer powder, primary particle size 21nm Anhydrous ethanol (CH3CH3OH), analytically pure; formaldehyde (HCHO), mass fraction of 37% to 40%.
1 . 2 test equipment
Sartorius electronic balance (maximum weighing 110g, accuracy 0.000lg); SY3100DH ultrasonic cleaner; DHG. 9123C automatic computer drying oven; TricolorP. A0/A1 rolling mill; Agilent Technologies 6890N gas chromatograph.
1 . 3 sample production route
Cotton fabric degumming and removal of a hydroxyapatite dispersion system, soaking, washing, drying, baking, l-TiO2 dispersion, soaking, one baking, one baking, one ultraviolet light irradiation, a sample performance test.
1 . 4 test process
1.4.1 Adsorption process of nano-hydroxyapatite
In order to improve the dispersibility of the nano-hydroxyapatite particles and the adsorption performance of the cotton fibers, the water with a relatively strong polarity is used as a dispersing agent for the nano-hydroxyapatite, and the pH of the dispersion system is adjusted to 12.30 with ammonia water. Oscillate for 10 min (oscillation frequency 55 kHz) in an ultrasonic cleaner at 40 ° C to make it evenly dispersed, and then soak the cotton fabric in a hydroxyapatite dispersion system at about 30 ° C for more than 12 h to fully adsorb between the two, with distilled water. The mechanically deposited nano-hydroxyapatite on the cotton fabric is washed away and then baked to bond the hydroxyapatite to the fabric fibers.
Baking conditions: On the basis of ensuring the bonding strength of hydroxyapatite and cotton fiber, it is necessary to prevent cotton fiber from being damaged. The cotton fabric is baked at 120 ° C for more than 5 h, and the fibers begin to yellow; above 150 ° C, the fibers will decompose. Baking l selected samples were baked at l20 °C for 4 h to promote the grafting of hydroxyapatite on cotton fibers; baking 2 was selected to be baked at 100 °C for 2 h to enhance the adsorption of TiO2 nanoparticles on cotton fibers. effect.
1.4.2 TiO2 deposition padding process
In order to prevent the aggregation of TiO2 nanoparticles and increase the adsorption amount of TiO2 on cotton fibers, it is necessary to optimize the mass fraction of nano-TiO2 dispersion reagent and TiO2.
1.4.2.1 Dispersion system It is preferred to disperse nano-TiO2 with anhydrous ethanol and water in different proportions (mass ratio: 0:1, 1:4, 1:2, 1:1). The whole system is at 40 °C. The constant temperature ultrasonic cleaner oscillates 10rain (oscillation frequency 55kHz), and the stratification is observed after standing for 5h.
1.4.2.2 TiO2 mass fraction is preferred. The cotton fabric is padded in a dispersion of TiO2 mass fraction of 1%, 2%, 3%, 4%, 5%, 6%, and the whole system is placed at 40 ° C. Oscillating for 10 min (oscillation frequency 55 kHz) in a constant temperature ultrasonic cleaner, three-dip three-rolling (pressure: 29.4 N/cm, rotation speed 600 r/min), once per padding, fabric and TiO2 dispersion system at 40 ° C constant temperature ultrasonic cleaner The mixture was shaken once, and finally the fabric was baked at 100 ° C for 2 h. According to the effect of fabric decomposition of formaldehyde, the best TiO2 mass fraction is selected.
1.4.3 Photocatalytic test
The photocatalytic performance of the finished fabric was evaluated by testing the amount of decomposition of formaldehyde in a given environment. Under the irradiation of ultraviolet light, formaldehyde is decomposed into CO2 and H20 under the catalysis of nano-TiO2, so the degree of decomposition of formaldehyde can be reflected according to the change of CO2 content in the test tube.
Test method: Paste the fabric to be tested with a size of 10 cm×1.5 cm on the inner wall of a 30 mL test tube, and then add a certain amount of formaldehyde to the test tube with a burette, and then seal the test tube, and use an ultraviolet lamp in the underground test room (at room temperature of about 20 ° C). (Intensity 10400 u W/cm2) was irradiated for 2 hours, and then 0.2 mL of gas in the test tube was separately taken. The peak area of ​​CO in the gas after decomposition was measured by gas chromatography to measure the decomposition effect of the fabric on formaldehyde.
Effect of formaldehyde concentration on photocatalysis: Photocatalytic tests were carried out by adding 1, 2, 3, and 4 drops of formaldehyde to the burette. According to the determination of 1 mL of formaldehyde, about 27 drops, that is, the amount of 1 drop of formaldehyde is about 1/27 mL.
Orthogonal test: Three conditions of dispersion system, TiO2 concentration and formaldehyde concentration were optimized. The three-factor three-level orthogonal test was used to carry out formaldehyde photocatalytic test. The catalytic decomposition effect of formaldehyde was used to determine the optimum process conditions for fabric finishing.
2 results and discussion
2 . 1 Establishment of a decentralized system
After the dispersion system was allowed to stand for 5 h, the stratification was observed: there was obvious stratification in the beaker with the mass ratio of anhydrous ethanol to water of 0:1, and there was no obvious stratification in other beakers. Mainly because water and TiO2 are both polar compounds. When interacting, it is easy to form hydrogen bonds through the hydroxyl groups of water molecules and combine with each other to form agglomeration and form precipitates and stratify; when distilled water is mixed After the proportion of ethanol, the polarity of the dispersant is weakened, the occurrence of agglomeration is reduced, the dispersion is relatively uniform, stable, and delamination is difficult. It is indicated that ethanol is a good dispersant for ultrafine TiO2 powder, which enables ultrafine particle TiO2 to form a stable dispersion in aqueous solution. Literature [4] also shows that ethanol is a good dispersant for ultrafine TiO2 powder. Ethanol can form a good solvation layer on the surface of TiO2 particles, so that ultrafine TiO2 particles can obtain good and stable dispersion in aqueous solution, but due to absolute ethanol. It has weak polarity, is easy to volatilize, has weak affinity for cotton fiber and its surface protective agent, and is not easy to swell cotton fiber. The use of a mixed solution of anhydrous ethanol and water as a solvent facilitates the deposition of TiO2 nanoparticles on the surface of cotton fibers.
The photocatalytic performance of functional cotton fabrics will be affected by the dispersion properties of TiO2 in aqueous solution. In order to determine the optimum ratio of anhydrous ethanol to water, the experiment was carried out by selecting m (anhydrous ethanol): m (water) = 1:4, 1:2, 1:1 as the dispersant factor in the orthogonal test. .
2 . 2 Establishment of TiO2 mass fraction
The mass fraction of TiO2 also has an effect on photocatalytic decomposition of formaldehyde. The results of literature [5] show that the higher the solid content of TiO in the dispersion system, the larger the loading of TiO2 on the carrier; the more times the padding is, the more TiO2 is loaded, but the loading is increased with the number of padding The increase is very slow; when dip coating with high solid content TiO2 dispersion solution, the supported TiO2 is unevenly distributed on the surface of the carrier, and local accumulation of TiO2 causes a decrease in photocatalytic effect and affects the hand of the fabric.
In order to test the effect of TiO2 concentration on the photocatalytic effect of the fabric in the dispersion system, the experiment selected m (anhydrous ethanol): m (water) = 1:4, and the mass fraction of TiO2 was 1%, 2%, 3%, 4%, 5%, 6% of the dispersion was used to finish the fabric, and then the formaldehyde decomposition test was carried out, and the peak area of ​​the CO in the decomposed gas was measured by gas chromatography to evaluate the finishing effect of the fabric. The results are shown in Table 1. 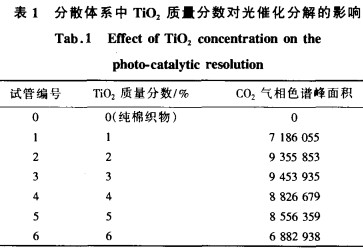
The experiment shows that when the mass fraction of TiO2 is very low, as the mass fraction of TiO2 increases (from 1% to 2%), the peak area of ​​CO and gas chromatogram increases significantly, that is, the photocatalytic effect of the sample is significantly enhanced. When the TiO mass fraction is 3%, the catalytic effect is the best. Continue to increase the mass fraction of TiO2 (from 3% to 6%), the photocatalytic activity of the sample decreased. This aspect is due to the agglomeration of nanoparticles in the dispersion system, so that the TiO2 particles adsorbed on the cotton fabric can not reach the nanometer level, which affects the catalytic effect of the fabric; on the other hand, when the TiO mass fraction is too high, the surface of the fabric The shielding power of TiO2 to light is increased, and the light can only reach the surface of the catalytic system, so that the photoexcitation efficiency of the internal TiO2 powder is lowered.
In the orthogonal test, the TiO mass fraction was selected at 3 levels of 2%, 3%, and 4%.
2 . 3 Effect of formaldehyde concentration Photocatalytic
The concentration of organic matter has an effect on the photocatalytic reaction effect. The reaction rate of photocatalytic oxidation can be described by the Langmuir-Hinshelwood kinetic equation:
r=kKC/(1+KC) (1)
Where: r is the reaction rate; C is the reactant concentration; K is the apparent adsorption equilibrium constant; k is the surface reaction rate constant of the photoactive surface active site.
When the concentration is low, KC "1, the dynamic equation can be simplified to
r=kKC=KC (2)
That is, when the initial concentration is low, the reaction rate is proportional to the concentration of the organic matter; when the concentration of the reactant increases to a certain extent, the reaction rate increases, but is not proportional; after the concentration reaches a certain limit, the reaction rate will not be affected.
In order to test the ability of the sample to catalytically decompose formaldehyde, the formaldehyde reaction concentration (different amount of formaldehyde was added) was tested.
In order to eliminate the interference caused by the decomposition of cotton fiber to form CO2, the test selected pure cotton fabric, using m (anhydrous ethanol): m (water) = 1:4 reagent as a dispersion, TiO2 mass fraction of 3% dispersion system The sample was tested for comparison. 1, 2, 3, 4, 5 drops of formaldehyde were added to the test tube, and after ultraviolet light (intensity of 10400 u W/cm) for 2 hours, the gas chromatographic test was carried out on the CO2 in the decomposed gas. The test results are shown in Table 2. Shown. 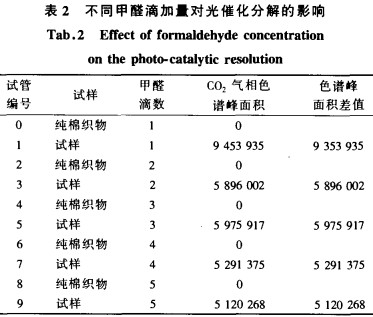
It can be seen from Table 2 that the peak area of ​​CO2 gas chromatograph of pure cotton fabric under ultraviolet irradiation is 0, indicating that the pure cotton fabric has almost no decomposition effect on formaldehyde; while the sample treated by TiO2 has a significant increase in the amount of CO2, indicating that the sample is formaldehyde. There is obvious photocatalysis. Further comparison with the test tubes No. 1, 3, 5, 7, and 9 shows that the decomposition effect is best when 1 drop of formaldehyde is added dropwise. With the increase of the number of formaldehyde drops, the decomposition effect of formaldehyde is not much different, that is, in the test tube space of 30 mL. It can significantly decompose 1/27mL of analytical pure formaldehyde, which is equivalent to decomposing 1234, 6mL of analytical pure formaldehyde in 1m space, indicating that the concentration of formaldehyde in the No. 1 tube has greatly exceeded the formaldehyde concentration in the actual living environment. According to formula (1), the decomposition effect of the sample cloth on formaldehyde in the actual living environment increases as the concentration of formaldehyde increases.
In the gas phase photocatalytic reaction, the photo-induced hole h + is more oxidizing than the ·OH, so that simple compounding of electron-hole pairs is suppressed as long as a suitable substance acts as a trapping agent for electrons and holes. The redox reaction can still occur. Under anhydrous conditions, the capture of photoelectrons can be oxygen adsorbed on the surface of the catalyst, and the trapping agent for photocavitations can be the organic matter itself. Therefore, the higher the formaldehyde concentration, the lower the oxygen concentration in the test tube, which reduces the adsorption of oxygen on the catalyst surface and reduces the efficiency of the catalytic reaction. The test results show that the decomposition effect is best when 1 drop of formaldehyde (about 1 2 7 mL) is added dropwise. In the orthogonal test, the amount of formaldehyde added was selected as 1, 2, and 3 drops as three levels.
2 . 4 orthogonal test
A three-factor three-level orthogonal test method was adopted. Nine test conditions were obtained by k(3) orthogonal method, as shown in Table 3. 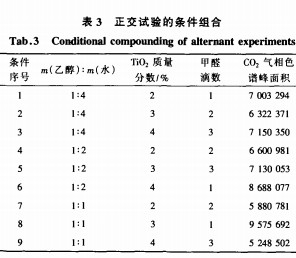
From the peak area of ​​the CO2 gas chromatogram in Table 3, when the mass ratio of the dispersant ethanol to water is 1:1, the mass fraction of TiO2 is 3%, and when 1 drop of formaldehyde is added, 10 cm×1.5 cm in a 30 mL test tube under ultraviolet irradiation. The gas chromatographic peak area of ​​CO2 produced by the decomposition of formaldehyde in fabrics can reach 9757692, which is much higher than other conditions, and can be considered as the best process for cotton fabric finishing.
3 conclusions
1) When TiO2 dispersion system is configured, adding a certain amount of absolute ethanol in water can reduce the aggregation of nanometers and form a stable dispersion. When the mass ratio of ethanol to water in the dispersant is 1:1, the dispersion effect of the system is the most it is good.
2) In the dispersion system, the higher the solid content of TiO2, the larger the loading of TiO2 on the fabric; but the content of TiO2 is too high, and the local accumulation of nanoparticles is easy to occur, which affects the photocatalytic effect and the hand of the fabric. When the mass fraction of TiO in the dispersion system is 3%, the photocatalytic effect of the fabric is the best.
3) Under certain process conditions, TiO2 deposited on the surface of the fabric can decompose a higher concentration of formaldehyde by ultraviolet irradiation.
Plastic Tube,Body Lotion Tube,Cosmetic Plastic Tube
Yiwu Dechao Glasses Firm , http://www.ywbeautyproduct.com
